Sneak Peak at Pharma Industry Survey on Observational Research: Progress not Perfection
May 8, 2015
By Jeff Trotter, President, Continuum Clinical
I write this blog the evening before giving a presentation on observational research to users of a popular clinical trials electronic data collection (EDC) system. This is a talk I’ve given easily a hundred times before to orient “traditional” clinical operations and data management professionals to the unfamiliar territory of a non-interventional study. I contrast controlled clinical trials with observational studies by employing some of my favorite vehicular metaphors: an 18-wheeler versus a sports car; the automotive test track versus actual traffic conditions; etc.
And the goal is always the same: to ensure my audience understands the fundamentally different purpose underlying observational research and, hence, the need for fundamentally different operational approaches.
Through multiple industry surveys over 6 years, we’ve tracked the trends in observational research. Now in its 9th edition, the Survey on Observational Research, a collaborative effort between Continuum Clinical and HealthEconomics.Com, seeks to shed light on key challenges and opportunities, with a special focus on the organizational constraints that often cause straightforward “real world” studies to be inadvertently over-engineered. This blog posting will provide a sneak peak at some of these findings.
While the Survey findings indicate general improvements in understanding and methodological consistency, there remain a variety of design and operational issues that still cause both squirming and eye-rolling, and indicate that we still have a ways to go before those commissioning and those implementing observational research see eye-to-eye.

Survey Says….
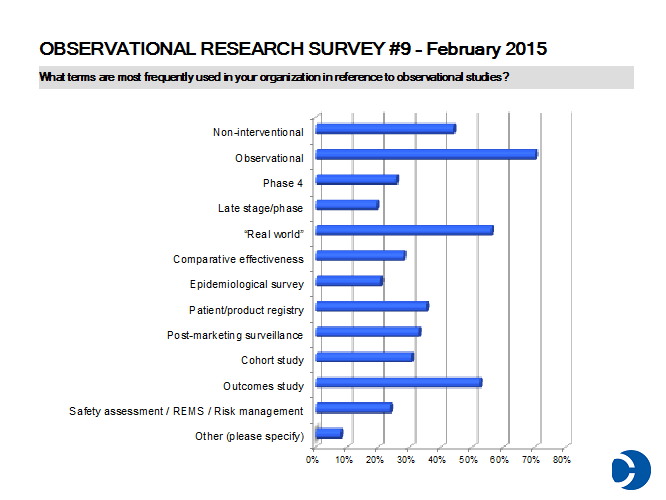
- There are 16 different monikers given to prospective, observational research (down from upwards of 25 in prior surveys)! This year’s award for most creative term supplied — “mystery shopping” — seems to reflect a particularly jaded view.
- Fairly consistent over the years is the proportion of observational studies conducted prior to product approval: 18%. Although in the minority, the importance of natural history and burden of disease studies remains strong.
- Health economics professionals believe their departments have responsibility for the funding, design, and implementation of observational studies at a far higher rate than do survey respondents from a variety of other departments. There is still inconsistency in terms of who is “driving the bus.
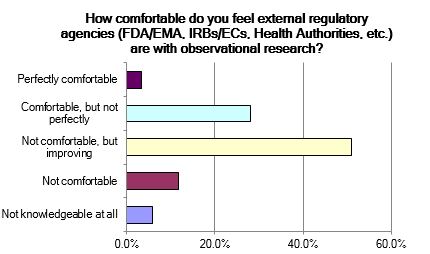
- There is a sense that regulatory authorities are becoming increasingly knowledgeable about observational research, but that they still often assess observational studies through an interventional “lens.”
- Up from recent years, nearly 50% of respondents indicate their organizations have Standard Operating Procedures for observational studies. Nearly two-thirds, however, feel that they are often “forced” to work with outside vendors that don’t really understand observational research, SOPs notwithstanding.
- A trend toward more direct-to-patient observational studies seems to be occurring due, potentially, to greater comfort with data acquired through advanced mobile technology. We’ll definitely be spending some time discussing this particular issue at the presentation of the Survey results. Indeed, if “traditional” clinical operations and data management professionals are still less-than-comfortable with observational studies, data generated through mobile-health applications will likely cause even greater levels of concern.
I look forward to presenting the findings from Survey #9, to exploring the implications, and to discussing issues we’ll need to address further in Survey #10! And, if you have a topic that you’d like to see addressed in the next iteration of the Survey on Observational Research, contact us at Continuum Clinical or leave a comment below.
Survey Findings on Observational Research at 2 PM on Monday, May 18th, 2015 at Loews Philadelphia Hotel (33rd Floor, Howe Room). Seating is limited. Please RSVP here.
Jeff Trotter is the President of Continuum Clinical, a company that brings together both clinical and commercial development in the areas of patient recruitment, Late-Stage research, health economics, and medical communications. He has been in the healthcare industry for over 30 years as an entrepreneur, researcher, consultant, and innovator. Jeff is an industry leader in the design and implementation of research studies generating “real world” evidence. He holds an MBA from Northwestern University and a bachelor’s degree in accounting from the University of Illinois.