Global Planning to Local Execution Market Success
October 16, 2023
Moving beyond static evidence development to ensure local market access success; responding to recent changes in governmental drug regulations and the role of automation
Written by Grammati Sarri and Radek Wasiak
The worldwide drug regulation landscape is rapidly changing. The introduction of the EU health technology assessment (HTA) Regulation (EU) 2021/2282 and US Inflation Reduction Act (IRA) created a need for pharmaceutical companies to develop strategies to generate data through diverse patient data sources and use complex methodologies while keeping pace with evolving evidentiary requirements for their products across healthcare decision-making bodies.
So, how can companies prepare for multiple, successful local submissions? Traditionally, sponsors have developed global value dossiers (GVD) including a core compendium of evidence to support the value story of new technologies, which are then tailored for national (local)-level HTA reviews or other payer interactions. However, the static, single-timepoint nature of GVDs and the often-disconnected activities supporting local submissions (evidence synthesis, economic analyses, and pricing negotiations) result in both duplicate efforts and disjointed value story messages across continents and countries.
The key question for sponsors is: Can companies still rely on GVDs to efficiently capture the abundance of clinical and economic data to meet the diverse and specific requirements for new regulations (such as joint clinical assessment and IRA) and for each local market?
Automation and artificial intelligence (AI) are increasingly recognized as integral parts of health economics and outcomes research activities; however, these tools have yet to be routinely considered as core activities in the preparation of evidence to support HTA activities and payer engagements.
We believe this innovative technology is becoming a necessity for sponsors to address the following emerging trends:
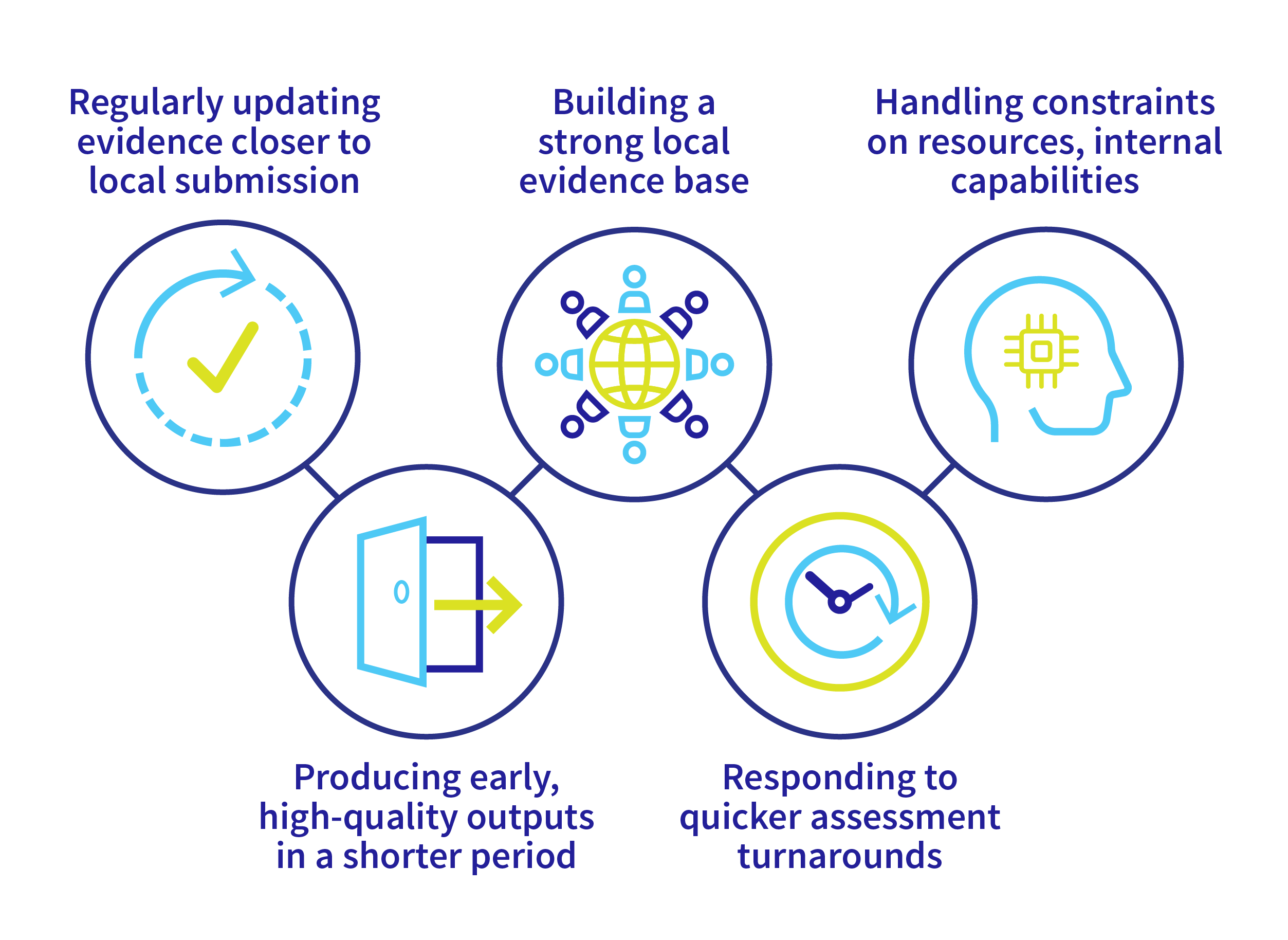
- While recent EU regulation requires clinical comparative assessments at the EU level, regularly updating evidence closer to local submissions along with meeting specific population, intervention/comparator, outcomes, and study design (PICOS) requests for each local market remain significant challenges for sponsors who only depend on traditional means of conducting systematic literature reviews (SLR) and comparative effectiveness assessments.
- Sponsors receive pressure from decision-makers to provide early data outputs for their products but based on higher evidentiary quality standards, such as using real-world data to ensure patient representation and equity concerns, and complex analytical approaches, all of which needs to be completed in a shorter period.
- A product’s value story may be improved by building a strong local evidence base through a comprehensive understanding of local issues. Messaging around the value story of products should be proactively monitored and updated with new evidence on better characterization of disease, unmet needs, and patient profiles, and should account for rapid changes in clinical practice due to the increased market entry of new products and the variety of patient and healthcare system outcomes.
- Technology assessment periods tend to become shorter; this requires companies to be proactive to respond quickly and comprehensively to decision-makers’ requests and be able to address complex methodological questions in more comprehensive analytical ways.
- Resources and internal capabilities constraints among decision-makers (such as the lack of AI expertise) would require sponsors to be able to transparently demonstrate the validity and accuracy of the submitted evidence including processes (screening, data extraction, open-source economic models) and methods (codes). Furthermore, the explosion of methodological guidance documents and the demand for advanced methodologies would require other steps of submission preparation to be less resource intensive to allow sponsors to divert time and resources toward addressing complex analytical scenarios.
This is where automation and AI through online platforms such as Cytel’s LiveSLR® products come to the rescue; these online tools use automation and were developed to adhere to higher standards requested by payers and HTA agencies. They can store and present the vast quantity of literature as part of a global evidence package, but can easily and transparently filter relevant, local evidence based on PICOS and geography. Methods to trigger updates can be done using AI tools (such as machine learning algorithms). Outputs of Cytel’s LiveSLR® online platform can be used by different sponsor local teams by using automated reports with customized study selection criteria meeting requests from different local affiliates and payers. By speeding up time-consuming steps in local submissions such as conducting or updating local SLRs, manufacturers can invest more time and resources in building stronger, value-based local market access submissions.







