Changing the Clinical Trial Paradigm for Rare and Orphan (R&O) Diseases using Real-World Evidence
April 9, 2019
Interview with Flora Sandra Siami, MPH. Vice President of Clinical Research, Regulatory Affairs, and Quality Assurance at HealthCore-NERI.


Dr. Peeples: Millions of people are affected by rare diseases worldwide, yet few treatments are available to address these diseases. What are some of the unique challenges that researchers face when designing a clinical trial for rare and orphan diseases?
Sandi Siami: There are many challenges unique to developing and testing treatments for rare and orphan diseases. First and foremost is understanding the natural history of the disease. Because there are a limited number of people with the disease/condition, physicians may not be aware of the most appropriate way to diagnose the condition. Even if the disease/condition is diagnosed, there may be limited clinical information available to understand the disease/condition outside of published case studies.
Once sufficient information is available about the natural history, then products can be developed to treat the disease/condition because there is some level of understanding of the disease, its mechanism of action, how it progresses, and the population it may affect, but the population is also heterogenous. However, this information is needed to design the clinical trial to define eligibility (i.e. inclusion/exclusion criteria), evaluation measures (i.e. study procedures), identify endpoints (i.e. outcomes), and lend to the statistical assumptions for the trial design/methodology.

At HealthCore-NERI, we’ve grappled with a number of these rare/orphan disease trials, and have gained substantial insight into challenges and solutions. One approach we’ve used is to arrange and execute state-of-the-science Consensus Conferences. We’ve held several of these for our clients. These are usually 1-2 day conferences with CME accreditation as put forth by the ACCME that focus on major topic areas, such as pathophysiology and diagnostic criteria, unmet need and available treatment, clinical management, and quality of life and health outcomes that related to internationally available treatment and management of the disease. These conferences present current evidence and discuss how the disease is currently treated and managed either in primary care or a specialty, as well as quality of life and potential psycho-social impact of the disease or its associated symptoms and provide guidance for appropriateness of establishing disease-specific treatment guideline and strategies. A white paper-style report from the conference is generated and submitted to a peer-reviewed publication in a relevant top-line journal. This would serve as the basis of evidence to overcome the above challenges.
Dr. Peeples: The Orphan Drug Act was able to put a spotlight on rare and orphan diseases, encouraging more research to address the unmet need of patients impacted by these diseases. However, traditional clinical trials remain challenged in addressing this need. How do you see the growth in large data sets and real-world evidence impacting the ability to improve the volume and efficiency of research studies focused on rare and orphan diseases?
Sandi Siami: Identifying investigators that treat a rare disease is a major challenge, in addition to identifying the patients themselves. Therefore, having access to large integrated data sets and the use of real-world evidence is essential, but also represents a major clinical trial paradigm shift. In the past we have used disease registries that collected information about rare/orphan/underserved/neglected diseases/conditions. These registries are designed to learn more about the natural history, diagnostic patterns, medical interventions, and outcomes in order to determine the types of treatments/interventions that will be suitable for that particular patient population. Our registries have included as few as 40 patients to as many as 19,000 patients in diseases from sickle cell anemia and thalassemia to pediatric cardiomyopathy and congenital heart disease.
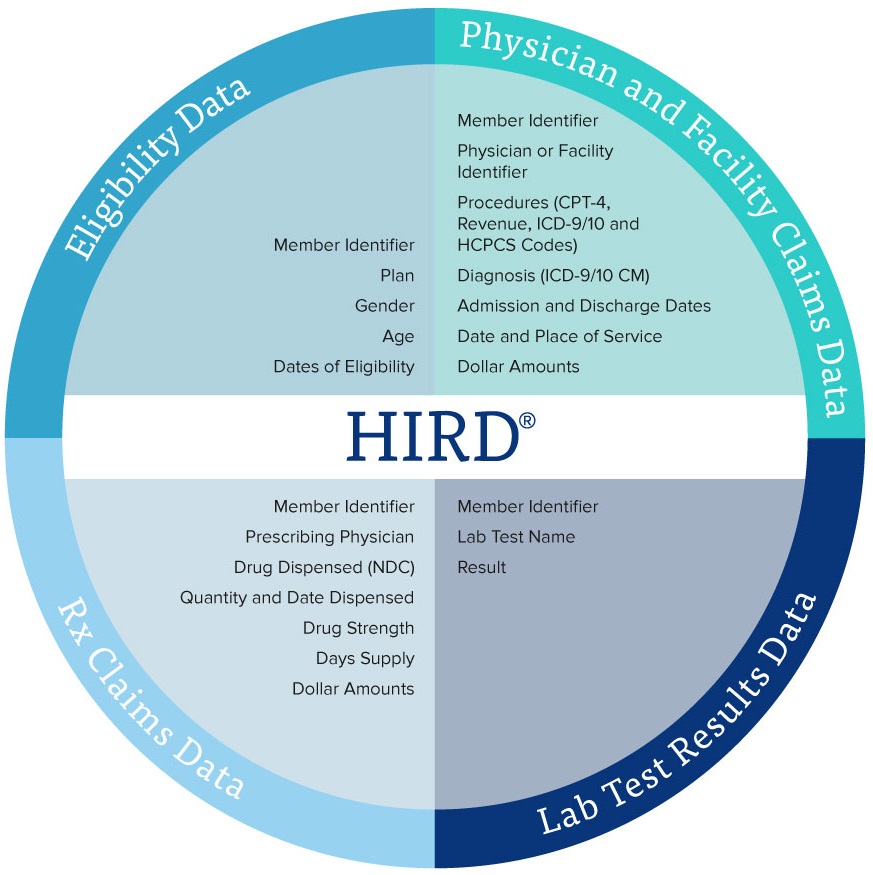
But in addition to registries and datasets, the HealthCore Integrated Research Database (HIRD®), for example, is a large administrative healthcare database maintained by HealthCore for use in health outcomes and pharmacoepidemiologic research. The HIRD is a broad, clinically rich, and geographically diverse spectrum of longitudinal medical and pharmacy claims data from health plan members across the U.S. Member enrollment, medical care (professional and facility claims), outpatient prescription drug use, outpatient laboratory test result data, and health care utilization may be tracked for health plan members in the database dating back to January 2006, and with diagnoses recorded in International Classification of Disease, Version 10 (ICD-10) since October 2015. The HealthCore Integrated Research Environment (HIRE) has the ability to link the claims data in the HIRD to complementary data sources, including inpatient and outpatient medical records, national vital statistics records, cancer and vaccine registries (state-by-state), disease and device registries, member and provider surveys, and point of care clinical data, which is especially critical for rare, orphan, and underserved diseases/conditions. Using these resources, HealthCore conducts real-world research designed to meet various client needs, including retrospective database studies, medical record review studies, cross-sectional and longitudinal patient and PRO surveys, electronic data linkage studies (including linkage of patient survey data with electronic claims), randomized controlled trials (RCTs), pragmatic clinical trials (PCTs), and site-based enrollment for prospective observational studies using electronic data capture.
Dr. Peeples: There has been substantial growth in interest and research around genetics and genomics. In many cases, genetic diseases are being described alongside rare and orphan diseases. Do you see genomics research as a way to further accelerate research for other rare and orphan diseases?
Sandi Siami: Genomics is an integral part of rare/orphan disease research and has been for over a decade. There are different approaches to genomic studies such as genome wide association, copy number variation, pathway analysis, and next generation studies, all of which have been used to identify genetic variants in the pediatric population. A great example is the Bench to Bassinet Program (B2B) funded by the National Heart, Lung, and Blood Institute, for which HealthCore-NERI served as the initial Coordinating Center. One of the studies, referred to as CHD GENES, collected phenotype and biospecimens from children, their biological parents, and siblings to determine the primary and secondary aims which include genome-wide association studies, whole exome sequencing, and whole genome sequencing to discover genes responsible for congenital heart disease. Secondary aims included identification of mutations responsible for CHD in large numbers of participants, and genotype/phenotype correlation including long-term clinical follow-up of enrolled participants to determine how genetics influences the clinical outcome in CHD.
Another example, for which HealthCore-NERI was a Coordinating Center is the Cooperative Study of Sickle Cell Disease that identified genetic variants associated to the severity of sickle cell disease and fetal hemoglobin expression. Results of these genomic studies can then be used to guide prospective clinical trials to sub-divide patient populations (by genotype/phenotype) to predict those that are responsive to some active drug substances or to use genetic variants as surrogates for diagnosis or outcomes. This is especially important as we are moving toward patient-centric personalized medicine.
Dr. Peeples: Similar to genomics research, special populations such as pediatrics are singled out as areas of importance in the R&O research world. Do you see this as a benefit in that it provides visibility for a broader class of research into R&O, or does this disadvantage other R&O diseases that may not be ‘trending’? On the other hand, how do you see R&O research benefiting such special populations?
Sandi Siami: Most rare diseases are also life-threatening or aggressively progressive in nature, and many also affect the pediatric population. Thus, once a potential therapy is targeted, identifying potential participants for clinical trials becomes challenging not only because of the rare nature of the disease and the limited number of physicians/investigators that may treat the disease but the special ethical considerations that must be given to children who are considered part of the vulnerable population.

More than half of the rare/orphan diseases/conditions affect the pediatric population, and as you know, research in this vulnerable population also has its own challenges. Certainly there are the standard regulatory pathways such as the Humanitarian Device Exemption, Accelerated Approval, Breakthrough Designation, or Regenerative Medicine Advanced Therapy Designation to reduce the regulatory, economic, legal, technical, and logistical burdens associated with development of interventions. And the additional regulations encouraging interventions in pediatric populations under the Best Pharmaceutical for Children Act (BPCA) and Pediatric Research Equity Act (PREA) may account for concentration in pediatric research over other populations. Section 529 of the Food, Drug, and Cosmetic Act (FDCA) specifically encourages development of new interventions for the prevention and treatment of certain rare pediatric diseases.
But the reality is that 50-75% of rare diseases begin in childhood1, and thus it would be expected that there would be more research centered around pediatric rare diseases. According to Children’s Wish Foundation International there are 7,000 different types of rare diseases, 80% are genetic in origin, 50% are children, and 30% of the children don’t live past the age of 5 years of age. Organizations such as this, as well as Global Genes and National Organization for Rare Disorders provide resources to patients, families, researchers, and clinicians on rare diseases. HealthCore-NERI has over 30 years of experience in conducting research in this challenging population engaging more than 490 sites globally and close to 25,000 pediatric subjects in diseases ranging from sickle cell disease, thalassemia, Marfan syndrome, pulmonary hypertension, and spinal muscular atrophy to name a few. Given the unique nuances in conducting pediatric research, on top of the challenges of rare/orphan diseases, our digital strategies have produced award-winning products to aid in educating children, their parents, and pediatricians on clinical trials and participating in clinical trials from websites, to DVDs/posters, YouTube videos, Facebook, video games, and national broadcast film.
Works Cited
- 1-Bavisetty S, Grody WW, Yazandi S. Emergence of pediatric rare diseases: review of present policies and opportunities for improvement. Rare Dis. 2013;1:e23579.

Flora Sandra Siami, MPH, is Vice President of Clinical Research, Regulatory Affairs, and Quality Assurance at HealthCore-NERI with close to 25 years of industry experience. She oversees the Clinical Research business unit that includes early and late phase trials, including pragmatic clinical trials, across all therapeutic areas with specific interest and passion in rare, orphan, and underserved diseases as well as pediatric and minority populations. As part of her clinical research oversight, she directs the pharmacovigilance/medical device safety activities including management of Data and Safety Monitoring Boards and Clinical Events/Endpoints Committees. She leads all domestic and international regulatory affairs activities in over 37 countries worldwide. She oversee the quality assurance team overseeing SOPs, internal/external and client/regulatory agency audits, and quality systems.