Preparing a Successful ICER Strategy: Lessons from the Frontlines
October 29, 2021


To answer these questions, Dr. Patti Peeples of HealthEconomics.Com sat down with Matt Sussman, Vice President of Modeling & Strategy Services at Panalgo to discuss recent changes in ICER’s value assessment approach and Panalgo’s ICER Evaluation Roadmap. This interview precedes a highly-anticipated multi-stakeholder virtual panel discussion “Preparing a Successful ICER Strategy: Lessons from the Frontlines” on November 17, 2021 featuring critical insights from senior leaders from Amgen, a former ICER Chief Scientific Officer, and Panalgo’s Sussman.
Patti: Matt, at Panalgo, you’ve spent a number of years advising manufacturers how to develop a well-conceived and executed ICER strategy. In 2020, ICER revised their value assessment framework. What are the three biggest changes that will impact manufacturers?
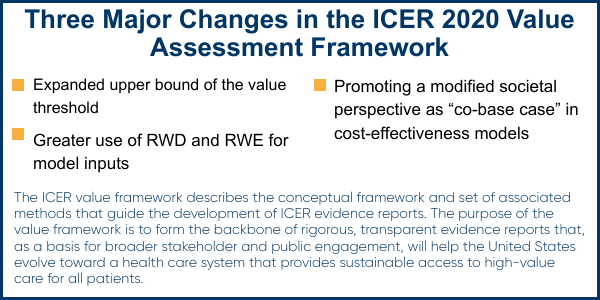
Matt: There were a number of updates in the 2020 Value Assessment Framework, but there are three changes that have the most impact for manufacturers. In general, I see these as a net positive for biopharma. These changes include expansion of the upper bounds of the cost-effectiveness threshold to $200,000 per Quality-Adjusted Life Year (QALY) from $150,000/QALY, augmented use of real world data (RWD) and real world evidence (RWE) for cost-effectiveness model inputs including prior evaluations, and promoting a modified societal perspective to “co-base case” in certain cost-effectiveness analyses when societal costs of care are large relative to the direct health care costs, and the impact of treatment on these costs is substantial. Learn more about these 2020 Value Assessment Framework changes in the video clip below.
Patti: What strategic changes are occurring within ICER to increase use of RWD/E in ongoing evaluations as well as prior assessments? How can the use cases for sickle cell disease assessment and hereditary angioedema assessment offer insights into ICER’s approach?
“For on-going evaluations, ICER has clearly demonstrated that it will use real-world evidence to populate selected model inputs.”
Matt: As an example of incorporating real world data into ongoing evaluations, ICER used a large administrative claims data set during its value assessment of treatments for sickle cell disease. The data set provided patient baseline characteristics, rates of treatment, and rates and costs associated with acute and chronic clinical events.
“For RWE-powered reassessments, ICER is focused on supplementing the comparatively limited evidence base that often accompanies accelerated approvals with RWE to provide a more comprehensive view of the drug’s comparative and cost-effectiveness.”
ICER piloted the use of RWE for reassessments of therapies that received accelerated approval from the U.S. Food and Drug Administration (FDA), and where RWE could be used to reduce uncertainty or improve comparative and cost-effectiveness. The pilot was limited to ICER evaluations reviewed in the past 24 months. Hereditary angioedema (HAE) was selected for the pilot because the original HAE economic model was very sensitive to small changes in assumptions of attack frequency, for on-demand treatment requirements, and prophylactic dosage regimens. The results from the pilot using observational RWE were used to generate new cost-effectiveness ratios and also new value-based prices (i.e., health benefit price benchmarks). We will discuss the RWE results, impact and manufacturer take-aways in more detail in our upcoming webinar.
Patti: Biopharma is increasingly called to identify knowledge gaps in clinical development programs, and incorporate RWE into their evidence base for HTA bodies and payers. What is your advice for critically appraising the use of RWD/E by ICER and other HTAs?
Matt: I have two recommendations. First, model inputs drive model results. It is essential to review ICER’s protocol for the model inputs and assess how these variables are being defined and validated. Second, replicate the analyses using alternative data analytic tools with the same or different data sources. In this video segment, Sussman describes how an important model input was defined in hereditary angioedema and explores how RWE was utilized by ICER.
Patti: Early preparation for HTA assessment, whether it be ICER or other bodies, is important and ideally this begins in Phase 2. On the other hand, a manufacturer may find themselves in the undesirable situation of initiating ICER preparation late in Phase 3. Regardless of timing, what success strategies can a manufacturer employ in their ICER engagement strategy?
Matt: ICER conducts many of its evaluations around product launch, and early preparation by manufacturers is one of the keys to maximizing success. Independent of the drug development stage, I recommend two essential components for optimalICER strategic engagement:
- Develop your own cost-effectiveness model so that you can anticipate ICER cost-effectiveness ratios and value-based pricing recommendations; and,
- Plan a mitigation strategy for internal and external communication, including coordinated HEOR studies, value proposition statements and objection management, all of which may be used in payer and provider-facing market access conversations or with internal stakeholders.
“Regardless of drug development stage, it’s really important to bring together different stakeholders to begin anticipating ICER model structure, assumptions, cost-effectiveness ratios, and value-based pricing recommendations.”
An ICER or HTA evaluation strategy begins with coordinating a diverse internal team that includes HEOR, Market Access, Commercialization, Medical Affairs, Epidemiology, and any other relevant internal stakeholders. The goal of this team is optimal preparation for the clinical and economic review, and it is essential that these viewpoints are considered when the manufacturer develops their substantiated response documents. Hear more from Sussman on ICER preparation here.
Patti: How can we learn more about successful ICER engagement strategies?
Matt: On Wednesday, November 17, 2021, Panalgo is sponsoring a webinar on Preparing a Successful ICER Strategy: Lessons from the Frontlines. The webinar features a knowledgeable and diverse panel of experts with three different perspectives on the ICER evaluation process: a manufacturer, consultant, and former ICER executive. We will take a deep dive into some critical issues that manufacturers grapple with every day, including:
- How to define success and set stakeholder expectations?
- Active engagement versus staying silent with ICER – what’s the best approach?
- How is RWE used to inform comparator selection when pivotal studies are often uncontrolled in accelerated approval scenarios?
- How is patient experience being considered?
- What should I know about ICER’s voting panel and why does it matter?

Panelists:
Anusha Kheir, MPH, Head US HTA Policy, Strategy and Engagement, Amgen
Dan Ollendorf, PhD, Director of Value Measurement and Global Health Initiatives at the Center for the Evaluation of Value and Risk in Health (CEVR)
Matt Sussman, MA, VP of Modeling & Strategy Services, Panalgo
Moderator:
Patti Peeples, RPh, PhD, President, HealthEconomics.Com, VP Evidence & Content, Scientist.com
It’s going to be a highly informative and engaging webinar, and I hope you’ll join. Meanwhile, if you have any questions, please contact our team at Panalgo.

Matt has a deep knowledge of ICER’s value assessment framework, best practices, and evaluations, and has led numerous strategic initiatives revolved around ICER assessments. He is a member of the International Society of Pharmacoeconomics and Outcomes Research (ISPOR) and is an executive committee member of the ISPOR Boston chapter. Matt received his undergraduate degree in Business Administration from the University of Michigan Ross School of Business and a graduate degree in Economics from Boston University.







